
Global Automated and Closed Cell Therapy Processing Systems Market Size by Type (Stem Cell and Non-stem Cell), Workflow (Separation, Expansion, Apheresis, Fill-finish, Cryopreservation, and Others), Scale (Pre-commercial and Commercial), End-users (Hospitals, Diagnostics Centers, Research Laboratories and Others), Regions, Segmentation, and Projection till 2028.
CAGR: 25.18% Current Market Size: USD 694.92 millionFastest Growing Region: APAC
Largest Market: North AmericaProjection Time: 2022-2028Base Year: 2020
Global Automated And Closed-Cell Therapy Processing Systems Market- Market Overview
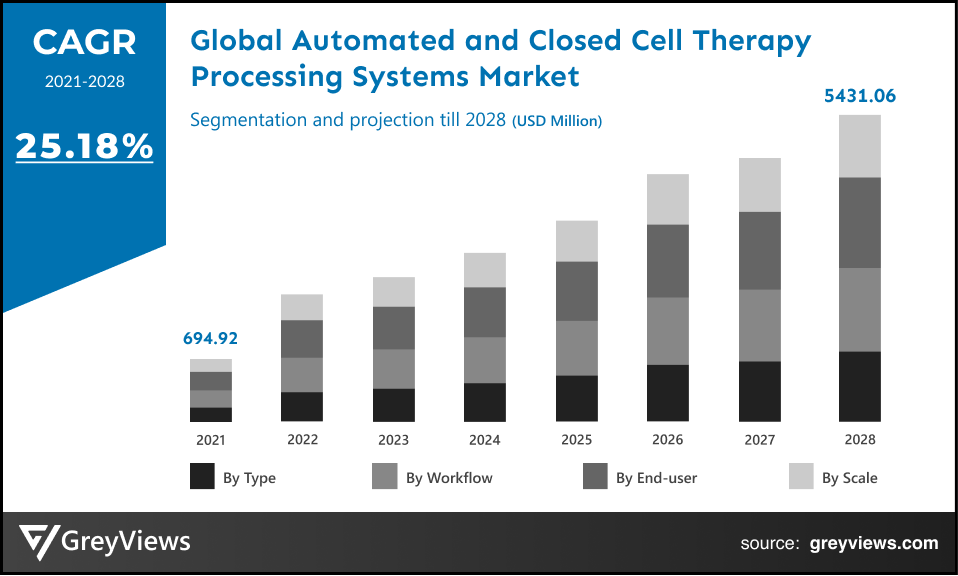
Global automated and closed-cell therapy processing systems market is expected to reach USD 5431.06 million by 2028, at a CAGR of 25.18% from 2022 to 2028. This growth of the automated and closed-cell therapy processing systems market is significantly driven by the rising demand for specific regeneration medicines. Automation technology offers several benefits through which these systems can be developed. The increasing requirement of automation in closed-cell therapy processing is fueling the growth of the market.
The commercialization of cell therapy requires the passing of stringent regulations at every stage. Cell therapy provides a way for the finding of various new regenerative medicines. The antibodies are manufactured using cell therapy processing systems. The manufacturing of cell and genes therapy is complex and thus requires intensive labor during the whole process. The processing of cells and genes requires various procedures and steps, including separation, expansion, apheresis, fill-finish, and preservation. Thus, in order to develop a successful cell therapy procedure, the use of automated systems is required. The use of automated and closed-cell therapy processing systems helps in conducting the cell therapy process safely and simply.
The automated and closed-cell therapy processing system are majorly used in commercial and clinical cell processing operations. The closed-cell therapy processing systems offer the highest protection against contamination. The presence of contamination can influence the test result, hence affecting the efficacy of the process. These automated systems can help in eliminating the delay in the therapy processing solutions when done by manual labor. It even reduces the requirement of a cleanroom space that is costlier to set as well as operate. With the help of automated and closed-cell therapy processing systems, multiple batches can be processed at once.
Request Sample:- Automated and Closed Cell Therapy Processing Systems Market
Market Dynamics
Drivers:
- The increasing popularity of regenerative medicine will boost the market growth
Regenerative medicines help in the repair and replacing of diseased as well as damaged cells. There are multiple processes that are used in the development of regenerative medicines. Tissue engineering is a crucial process for the development of regenerative medicine. The organs which are either damaged or are suffering from some disease can be easily replaced with the help of regenerative medicine. The development of regenerative medicine is expected to propel the demand for automated and closed-cell therapy processing systems.
- Automation of cell processing technologies
The cell therapy processing technologies require the use of automated systems in order to speed up the process and eliminate any delay which is usually associated with the manual processes. The automated and closed-cell therapy processing systems are gaining popularity in cell therapy applications owing to the integration of several processes and reduced complexity. With the help of an automation system, the probability of cross-contamination is almost reduced to zero.
Restraints:
- High capital cost
The installation cost of automated and closed-cell therapy processing systems is expensive, and thus it cannot be afforded by many medium and small-scale consumers. There are too many features in the closed-cell therapy processing systems, which makes it costlier. Further, the maintenance cost adds up to the total cost.
Opportunities:
- The rise in investments in clinical trials
The increasing incidence of chronic diseases has led to an increase in the number of clinical trials for various medicines. The rising number of demands of clinical trials has further provided lucrative opportunities for the market. Recently, the use of automated and closed-cell therapy processing systems has been gaining popularity in pre-commercial as well as commercial clinical trials. The rising number of clinical trials requires efficient call handling abilities while simultaneously reducing the probability of any error.
Challenges:
- Imposition of stringent regulations
The automated and closed-cell therapy processing systems require the passing of stringent regulations in order to be launched in the market. This system helps in the processing of cells, which can be further used in the development of regenerative therapies. Thus, the approval of closed-cell therapy processing systems requires the passing of several stages in order to be proved safe for the required applications.
Segmentation Analysis
The global automated and closed-cell therapy processing systems market has been segmented based on type, workflow, scale, end-users, and regions.
By Type
The type segment includes stem cells and non-stem cells. The non-stem cell segment led the automated and closed-cell therapy processing systems market with a market share of around 63.25% in 2021. This segment's growth is mainly attributed to the fact that they are mainly being preferred in cell therapy processing due to the rising number of product launches. There are various research projects centered on non-stem cells, and thus the demand for automated systems is increasing for non-stem cell types.
By Workflow
The workflow segment includes separation, expansion, apheresis, fill-finish, cryopreservation, and others. The expansion segment led the automated and closed-cell therapy processing systems market with a market share of around 29.32% in 2021. The expansion application is used for different cellular experiments as it is helpful in the research of regenerative medicines. Expansion processes can be centralized using automated and closed-cell therapy processing systems.
By Scale
The scale segment includes pre-commercial and commercial. The pre-commercial segment led the automated and closed-cell therapy processing systems market with a market share of around 74.92% in 2021. Pre-commercial activities include all those processes that are done before launching the product in the market. Thus, the majority of the companies are installing such software in order to gain efficacy in pre-commercial activities. Pre-commercial activities should test the efficacy of the end product in order to be proved successful for the administration of consumers. Therefore, the use of such systems helps in determining the success of the medicine.
By End-user
The end-user segment includes hospitals, diagnostics centers, research laboratories, and others. The research laboratories segment led the automated and closed-cell therapy processing systems market with a market share of around 46.21% in 2021. The research laboratories are involved in the manufacturing of various regenerative medicines and the use of automated and closed-cell processing systems. These systems help in providing faster results and also process a greater number of batches at once.
By Regional Analysis
The regions analyzed for the automated and closed-cell therapy processing system market include North America, Europe, South America, Asia Pacific, and the Middle East, and Africa. North America region dominated the automated and closed-cell therapy processing market and held the 47.15% share of the market revenue in 2021.
- The presence of established diagnostic and research laboratories and healthcare systems is one of the primary reasons for the development of automated and closed-cell therapy processing systems. The production and precision technologies are more sought upon in the region due to the increasing research on regenerative medicines. The availability of advanced technologies in the healthcare sector is propelling new growth opportunities for the market.
- The Asia-Pacific region is likely to register the highest growth during the Projection period due to the rising demand for cell processing due to the increasing number of investments. Moreover, China and Japan are some of the key countries for the automated and closed-cell therapy processing systems market in the Asia-Pacific region. Further, the rising healthcare spending is fueling up the market growth.
Key Industry Players Analysis
To increase their market position in the global automated and closed-cell therapy processing system business, top companies are focusing on tactics such as adopting new technology, mergers & acquisitions, product developments, collaborations, and partnerships, joint ventures, etc.
- Cellares Inc.
- Lonza
- Cytiva
- Terumo Corporation
- Thermo Fisher Scientific Inc.
- BioSpherix Inc.
- MiltenyiBiotec
- Fresenius Kabi
- Sartorius AG
- ThermoGenesis Holdings Inc.
Latest Development
- February 2021- Brooks Life Science and Cytiva had agreed to extend their capabilities in the automated cold chain. These two companies are working in order to gain expertise in the cryogen cold chain system of Cytiva.
- October 2018- Lonza announced the acquisition of a significant stake in Octane Biotech. Lonza had planned to acquire complete ownership in the company with the objective of developing automated technology for the requirement of scalable autologous. However, Octane Biotech had planned to maintain its exclusivity for the indication of orthopedic clinical in the Cocoon system.
Scope of the Report
Global Automated and Closed Cell Therapy Processing Systems Market by Type:
- Stem Cell
- Non-stem Cell
Global Automated and Closed Cell Therapy Processing Systems Market by Workflow:
- Separation
- Expansion
- Apheresis
- Fill-finish
- Cryopreservation
- Others
Global Automated and Closed Cell Therapy Processing Systems Market by Scale:
- Pre-commercial
- Commercial
Global Automated and Closed Cell Therapy Processing Systems Market by End-user:
- Hospitals
- Diagnostics Centers
- Research Laboratories
- Others
Global Automated and Closed Cell Therapy Processing Systems Market by Region:
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Russia
- Asia-Pacific
- Japan
- China
- India
- Korea
- Southeast Asia
- South America
- Brazil
- Peru
- Middle East and Africa
- UAE
- South Africa
- Saudi Arabia
Frequently Asked Questions
What is the market size of the automated and closed-cell therapy processing system Market?
Global automated and closed-cell therapy processing system market is expected to reach USD 5431.06 million by 2028, at a CAGR of 25.18% from 2022 to 2028.
Which regions have been studied for the regional analysis of the global automated and closed-cell therapy processing system market?
The regions analyzed for the automated and closed-cell therapy processing system market include North America, Europe, South America, Asia Pacific, and the Middle East, and Africa.
What is the segmentation considered for the analysis of the global automated and closed-cell therapy processing system market?
The global automated and closed-cell therapy processing system market has been segmented based on type, workflow, scale, end-user, and regions
What is the key driver of the automated and closed-cell therapy processing system market?
Rising demand for low maintenance, highly efficient, and versatile automated cell therapy processing system in the molecular biology application, is primarily driving the growth of the automated and closed-cell therapy processing system market.
Which are the leading market players active in the automated and closed-cell therapy processing system market?
Leading market players active in the global automated and closed-cell therapy processing system market are Cellares Inc., Lonza, Cytiva, Terumo Corporation, Thermo Fisher Scientific Inc., BioSpherix Inc., MiltenyiBiotec, Fresenius Kabi, Sartorius AG, ThermoGenesis Holdings Inc. among others.
What are the ongoing trends that are projected to influence the market in the upcoming years?
Manufacturers in the automated and closed-cell therapy processing system market are using advanced production technologies to incorporate better results, installation, and performance of the whole process. In addition, the rising number of investments will contribute to the demand for automated and closed-cell therapy processing systems.
What are the detailed impacts of the COVID-19 pandemic on the global market?
The pandemic has significantly affected several industries and has caused a worldwide economic slowdown. In order to stop the spread of the virus, the development of vaccines was the need of the hour. The automated and closed-cell therapy processing systems have risen in recent times, owing to the requirement for the preparation of vaccines. The research and diagnostics laboratories are deploying huge investments in automated systems, thus, creating demand for the automated and closed-cell therapy processing system.
How the company profile has been selected?
Based on the sales revenue, product offering, and regional presence, the companies are selected.
Political- The automated and closed-cell therapy processing systems require the passing of stringent regulations in order to be launched in the market. This system helps in the processing of cells, which can be further used in the development of regenerative therapies. Thus, the approval of closed-cell therapy processing systems requires the passing of several stages in order to be proved safe for the required applications.
Economic- The increasing incidence of chronic diseases has led to an increase in the number of clinical trials for various medicines. The rising number of demands of clinical trials has further provided lucrative opportunities for the market. Recently, the use of automated and closed-cell therapy processing systems has been gaining popularity in pre-commercial as well as commercial clinical trials. The rising number of clinical trials requires efficient call handling abilities while simultaneously reducing the probability of any error. The installation cost of automated and closed-cell therapy processing systems is expensive, and thus it cannot be afforded by many medium and small-scale consumers. There are too many features in the closed-cell therapy processing systems, which makes it costlier. Further, the maintenance cost adds up to the total cost.
Social- The Asia-Pacific region is likely to register the highest growth during the Projection period due to the rising demand for cell processing due to the increasing number of investments. Moreover, China and Japan are some of the key countries for the automated and closed-cell therapy processing systems market in the Asia-Pacific region. Further, the rising healthcare spending is fueling up the market growth.
Technological- Regenerative medicines help in the repair and replacing of diseased as well as damaged cells. There are multiple processes that are used in the development of regenerative medicines. Tissue engineering is a crucial process for the development of regenerative medicine. The organs which are either damaged or are suffering from some disease can be easily replaced with the help of regenerative medicine. The development of regenerative medicine is expected to propel the demand for automated and closed-cell therapy processing systems.
Environmental- Because of the increasing pollution and changing lifestyle several new diseases are taking place. Many of these diseases are deadly and can take the life of humans. In order to fight these diseases several treatments have been developed which helps in treating these diseases. Government is also taking up stringent actions to fight the pollution and keep the environment clean and green.
Legal- Lonza announced the acquisition of a significant stake in Octane Biotech. Lonza had planned to acquire complete ownership in the company with the objective of developing automated technology for the requirement of scalable autologous. However, Octane Biotech had planned to maintain its exclusivity for the indication of orthopedic clinical in the Cocoon system.
- Introduction
- Objective Of The Study
- Overview Of Automated and Closed Cell Therapy Processing System Market
- Markets Covered
- Geographic Scope
- Years Considered For The Study
- Currency And Pricing
- Executive Summary
- Premium Insights
- Market Attractiveness Analysis
- Market Attractiveness Analysis By Type
- Market Attractiveness Analysis By Workflow
- Market Attractiveness Analysis By Scale
- Market Attractiveness Analysis By End-user
- Market Attractiveness Analysis By Region
- Industry SWOT
- Industry Trends
- Porter’s Five Forces Analysis
- Country Level Analysis
- Factors Considered For The Study
- Pointers Covered At Macro Level
- Pointers Covered At Micro Level
- Year On Year Growth Rate
- Technology Road Map
- Market Attractiveness Analysis
- Market Overview and Key Dynamics
- Drivers
- Restraints
- Opportunities
- Challenges
- Global Automated And Closed Cell Therapy Processing System Market Analysis and Projection, By Type
- Segment Overview
- Stem Cell
- Non-stem Cell
- Global Automated And Closed Cell Therapy Processing System Market Analysis and Projection, By Workflow
- Segment Overview
- Separation
- Expansion
- Fill-finish
- Cryopreservation
- Others
- Global Automated And Closed Cell Therapy Processing System Market Analysis and Projection, By Scale
- Segment Overview
- Pre-commercial
- Commercial
- Global Automated And Closed Cell Therapy Processing System Market Analysis and Projection, By End-user
- Segment Overview
- Hospitals
- Diagnostics Centers
- Research Laboratories
- Others
- Global Automated And Closed Cell Therapy Processing System Market Analysis and Projection, By Regional Analysis
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Asia-Pacific
- Japan
- China
- India
- South America
- Brazil
- Middle East and Africa
- UAE
- South Africa
- North America
- Global Automated And Closed Cell Therapy Processing System Market-Competitive Landscape
- Overview
- Market Share of Key Players in Global Automated And Closed Cell Therapy Processing System Market
- Global Company Market Share
- North America Company Market Share
- Europe Company Market Share
- APAC Company Market Share
- Competitive Situations and Trends
- Product Launches and Developments
- Partnerships, Collaborations and Agreements
- Mergers & Acquisitions
- Expansions
- Company Profiles
- Cellares Inc.
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Cytiva
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Terumo Corporation
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Thermo Fisher Scientific Inc.
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- BioSpherix Inc.
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- MiltenyiBiotec
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Fresenius Kabi
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Sartorius AG
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- ThermoGenesis Holdings Inc
- Business Overview
- Company Snapshot
- Company Market Share Analysis
- Company Product Portfolio
- Recent Developments
- SWOT Analysis
- Cellares Inc.
List of Table
- Global Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Global Stem Cell, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Non-stem Cell, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Global Separation, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Expansion, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Apheresis, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Fill-finish, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Cryopreservation, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Others, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Global Pre-commercial, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Commercial, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Global Hospitals, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Diagnostics Centers, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Research Laboratories, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- Global Others, Automated And Closed Cell Therapy Processing System Market, By Region, 2021-2028 (USD Million)
- North America Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- North America Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- North America Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- North America Automated And Closed Cell Therapy Processing System Market, By End-user 2021-2028 (USD Million)
- US Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- US Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- US Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- US Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Canada Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Canada Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Canada Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Canada Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Mexico Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Mexico Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Mexico Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Mexico Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Europe Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Europe Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Europe Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Europe Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Germany Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Germany Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Germany Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Germany Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- France Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- France Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- France Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- France Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- UK Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- UK Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- UK Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- UK Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Italy Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Italy Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Italy Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Italy Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Spain Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Spain Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Spain Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Spain Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Asia Pacific Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Asia Pacific Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Asia Pacific Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Asia Pacific Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Japan Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Japan Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Japan Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Japan Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- China Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- China Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- China Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- China Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- India Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- India Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- India Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- India Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- South America Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- South America Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- South America Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- South America Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Brazil Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Brazil Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Brazil Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Brazil Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- Middle East and Africa Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- Middle East and Africa Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- Middle East and Africa Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- Middle East and Africa Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- UAE Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- UAE Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- UAE Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- UAE Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
- South Africa Automated And Closed Cell Therapy Processing System Market, By Type, 2021-2028 (USD Million)
- South Africa Automated And Closed Cell Therapy Processing System Market, By Workflow, 2021-2028 (USD Million)
- South Africa Automated And Closed Cell Therapy Processing System Market, By Scale, 2021-2028 (USD Million)
- South Africa Automated And Closed Cell Therapy Processing System Market, By End-user, 2021-2028 (USD Million)
List of Figures
- Global Automated And Closed Cell Therapy Processing System Market Segmentation
- Global Automated And Closed Cell Therapy Processing System Market: Research Methodology
- Market Size Estimation Methodology: Bottom-Up Approach
- Market Size Estimation Methodology: Top-Down Approach
- Data Triangulation
- Porter’s Five Forces Analysis
- Value Chain Analysis
- Global Automated And Closed Cell Therapy Processing System Market Attractiveness Analysis By Type
- Global Automated And Closed Cell Therapy Processing System Market Attractiveness Analysis By Workflow
- Global Automated And Closed Cell Therapy Processing System Market Attractiveness Analysis By Scale
- Global Automated And Closed Cell Therapy Processing System Market Attractiveness Analysis By End-user
- Global Automated And Closed Cell Therapy Processing System Market Attractiveness Analysis By Region
- Global Automated And Closed Cell Therapy Processing System Market: Dynamics
- Global Automated And Closed Cell Therapy Processing System Market Share By Type (2021 & 2028)
- Global Automated And Closed Cell Therapy Processing System Market Share By Workflow (2021 & 2028)
- Global Automated And Closed Cell Therapy Processing System Market Share By Scale (2021 & 2028)
- Global Automated And Closed Cell Therapy Processing System Market Share By End-user (2021 & 2028)
- Global Automated And Closed Cell Therapy Processing System Market Share By Regions (2021 & 2028)
- Global Automated And Closed Cell Therapy Processing System Market Share By Company (2021)


